Team 1 : Mononuclear phagocytes, Immunopathology, Immunovirology
Research program
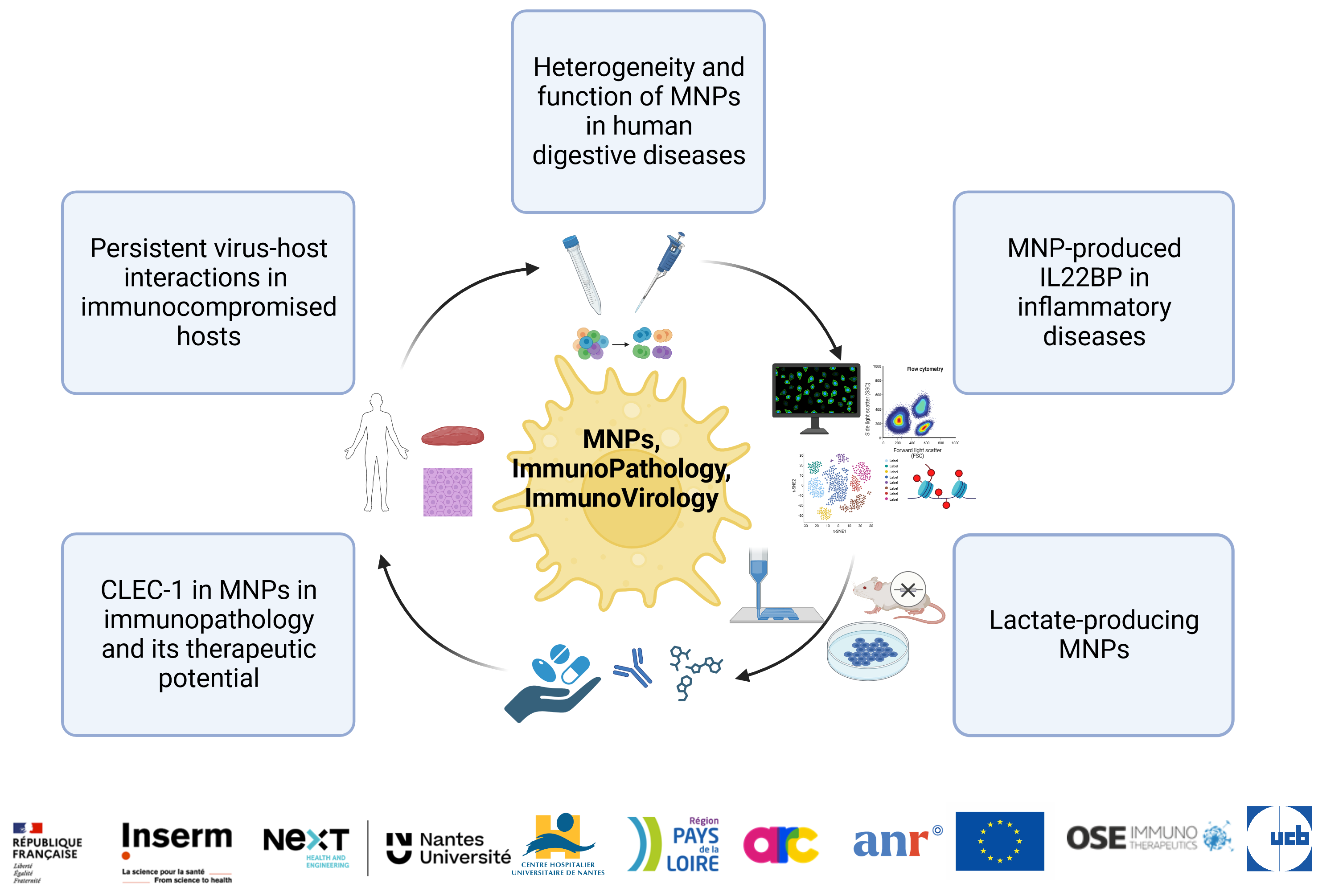
Two major axes of research are developed in Team 1 :
1. Biology of Mononuclear Phagocytes (MNP) in Immuno-Pathology.
Objectives:
Mononuclear phagocytes (MNPs), by bridging innate and adaptive immunity, play important roles in pathological processes within tissues. We aim to unravel the heterogeneity as well as pathogenic/immunoregulatory functions of these cells in different human diseases such as inflammatory bowel diseases, cancer and infection in order to better understand these pathologies and identify new therapeutics for clinical transfer.
Ongoing projects:
Project #1: Dissecting MNP Heterogeneity in Crohn’s Disease (CD) (PI J. Martin)
In CD inflamed ileums, we identified a GIMATS (IgGplasma cells, inflammatory MNP (inf.MNP), activated T and stromal cells) response in a subgroup of patients with poor clinical response to anti-TNF therapy (Martin et al., Cell 2019). We aim to apply high dimensional single cell approaches to explore the MNP compartment in CD and characterize programs specifically driving inflammatory MNP generation and cellular interactions (ANR JC CDMNP (2020-2024), iSITE NexT Junior talent (2020-2023)).
Project #2: Heterogeneity of MNP and functions in pre-cancerous inflammatory gastric lesions (PI J. Martin)
Using high dimensional approaches, we aim to understand how the MNP molecular landscape is progressively shaped to promote tumor growth along the stepwise progression of precancerous lesions to gastric tumors. Ultimately, we aim at identifying early markers of disease at premalignant stages, and propose new therapeutic opportunities to help prevent gastric cancer (Fondation ARC (2021-2022) EU AIDA (2023-2027)).
Project #3: Role of lactate-producing MNPs (PI A. Moreau)
We recently demonstrated the safety of cell therapy using in vitro-derived tolerogenic MNP in a Phase I/II clinical trial in kidney transplantation (Moreau et al. Kidney Int. 2023). Originally, these MNP are highly glycolytic cells and their high production of lactate contributes to the inhibition of CD4+ T cell proliferation and cytokine production in vitro (Marin et al. Cell Metabolism 2019). Since a reduction of CD8 T cell activation and an increase of Foxp3 expression were observed in MNP-treated patients, we now aim to investigate the mechanisms of action of these lactate-producing MNP on the regulation of these T cell populations to improve the clinical protocol for a future clinical trial (EU Instruct (2020-2023), FRM (2023-2025), SFNDT (2023-2024)). We also aim to investigate whether a similar population of lactate-producing MNP with regulatory functions is present in humans, notably in tumors containing a high lactate concentration (Labex IGO (2020-2023), Fondation ARC (2023-2024)).
Project #4: The regulation of IL-22 by MNP-produced IL-22BP (PI: R Josien)
We previously showed that IL-22BP is produced by subsets of DC and macrophages (Fantou et al. Front Immunol 2022) and we aim to fully characterize the phenotype, location as well as gene expression at the single cell level of IL-22BP producing MNP. Furthermore, as IL-22BP is upregulated in the skin of atopic dermatitis patients (Martin et al. J Immunol 2017), we aim in collaboration with UCB pharma and Dr. S Barbarot (CHU Nantes) to evaluate whether skin IL-22BP expression could define clinically relevant subgroups of patients (Région PdL (2018-2021), UCB (2020-2023)).
Project #5: CLEC-1 in MNPs : immunopathology and therapeutic potential (PI: E Chiffoleau)
We recently showed that the innate C-type Lectin receptor CLEC-1 is a receptor of necrotic cells and acts as an immune checkpoint by limiting the cross-presentation of dead cell-associated antigens by cDC1 to CD8+ T cells (Drouin et al. Sci Adv 2022). We now aim to study in depth the molecular mechanisms of CLEC-1 in MNPs and cDC1 cross-presentation (cytokine and chemokine expression, phagosome maturation). Moreover, we aim to determine the role of CLEC-1 in MNP-induced immunosuppression in the context of cancer (partnership OseImmunotherapeutics) and sepsis (collaboration C Jacqueline/J Poschmann team 6) and determine the therapeutic value and mechanisms of human CLEC-1 antagonists in preclinical models (ANR PRCE 2019-2023, ANR PRC 2023-2026, Région PdL (2023-2024), Fondation ARC 2023-2024, ANRT CIFRE OSEI (2023-2026)).
2. Host-pathogen interactions of persistent human viruses.
Objectives:
Persistent human DNA viruses, such as human herpesviruses, polyomaviruses, or anelloviruses, represent an important part of the human virome. When the equilibrium between the virus and the immune system is altered, uncontrolled viral replication can occur, which, in hosts whose immune systems are compromised, may lead to severe disease. We aim to understand the cellular and molecular mechanisms of host-virus interactions, and the impact on innate and adaptive immune responses on persistent human virus infections in immunocompromised hosts.Ongoing projects:
Project #6: Virus host interactions and viral evolution during BKPyV infections (PI: C Bressollette-Bodin)
We aim to identify host and viral factors associated with the control of BKPyV infections after kidney transplantation. We previously observed the occurrence of mutations in the VP1 gene coding for the major capsid protein, and identified mutational signatures related to APOBEC3A/B activity but also an additional, as yet unidentified pathway (McIlroy et al, Viruses 2022). We now want to complete the study of intra-individual viral evolution, and compare controlled versus uncontrolled infections. We have developed whole genome sequence analysis strategies that combine long molecule and overlapping amplicon sequencing. On the host side, GWAS and KIR/HLA analysis in donor/recipient pairs of a large cohort is ongoing to identify genetic factors associated with the infection (collaboration S. Limou, N. Vince, team 3) (ANR PRC 2017-2022, AO Recherche et Greffe ABM 2022).Project #7: Polyomavirus capsid interactions with antibodies and host-cell receptors (PI: D McIlroy)
The major polyomavirus (PyV) capsid protein, VP1, shows very little variation between patients and also seems genetically stable over time. Nevertheless, in kidney transplant patients with persistent BKPyV replication, the VP1 protein accumulates mutations, which we showed impact both neutralisation escape (McIlroy et al. Viruses 2020) and the interaction with host cell receptors (Sorin et al. Cell Reports 2023). We also characterized several broadly-neutralizing monoclonal antibodies active against all four BKPyV genotypes as well as most neutralization escape variants (Nguyen NK, Life Sc Alliance 2023). Current projects aim to evaluate the B-cell repertoire against BKPyV, the closely related JCPyV, and human papillomaviruses, and to identify alternative host receptors for BKPyV entry. (Région PdL 2019-2024; ANR PRC “Rep2Eps” 2023-27)Project #8: In vitro 3D models to study the contribution of MNPs to the BKPyV infection (PI: F Halary)
We identified blood and renal MNPs, mostly cDC2s, as probable key players in the capture and spread of BKPyV virions. These cells are also capable of protecting virions from neutralization (Sikorski et al., PLoS Pathogens 2021). We are now investigating the mechanics behind the BKPyV infectious process, involving both MNPs and stromal or tubular cells by two technological approaches: spatial transcriptomics to explore the post-transplant BKPyV-reactivating kidney and the organ-on-chip to model the proximal kidney tubule, a major site for BKPyV reactivation in vivo (BIRDIE Consortium, project No 964452, ERC Pathfinder, 2021-2025).Project #9: The respiratory virome of critically ill patients (PI: C. Bressollette-Bodin)
In critically ill patients, we are investigating the complex relationships between and the virome composition and immune disorders observed during intensive care unit hospitalisation (collaboration A. Roquilly, J. Poschmann, team 6). We first showed that HSV reactivations were associated with a specific monocyte transcriptomic signature and poor recovery in brain-injured patients (Chaumette et al, AJRCCM 2022). In the same cohort, we show variations of Torque Teno Virus viral loads during the ICU stay in association with hospital acquired pneumonia and acute respiratory distress syndrome. The composition of the respiratory virome (including bacteriophages) is currently being studied using shot gun metagenomics (collaboration L. Josset, HCL Lyon). (H2020-SC1-2019 HAP2).
Clinicians / Associate Researchers
Research assistants
Martin BRAUD - IE
Cécile BRAUDEAU - IHP
Flora COULON - AI
Alison DUMONT - IE
Manon LOIRAT - AI
Cécile PELTIER - IHP
Mathieu ROUEL - IE
Postdoctoral fellows
Selected publications
 Structural and functional analysis of natural capsid variants suggests sialic acid-independent entry of BK polyomavirus. Cell Rep. 2023;42(2):112114. PMID: 36790933
Structural and functional analysis of natural capsid variants suggests sialic acid-independent entry of BK polyomavirus. Cell Rep. 2023;42(2):112114. PMID: 36790933A Phase I/IIa study of autologous tolerogenic dendritic cells immunotherapy in kidney transplant recipients. Kidney Int. 2023 Mar;103(3):627-637. PMID: 36306921
Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med. 2022 Apr;28(4):766-779. PMID: 35190725
CLEC-1 is a death sensor that limits antigen cross-presentation by dendritic cells and represents a target for cancer immunotherapy. Sci Adv. 2022 Nov 16;8(46):eabo7621. PMID: 36399563
Monocyte Signature Associated with Herpes Simplex Virus Reactivation and Neurological Recovery after Brain Injury. Am J Respir Crit Care Med. 2022 Aug 1;206(3):295-310. PMID: 35486851